Judul : Blood In Hypertonic Solution
link : Blood In Hypertonic Solution
Blood In Hypertonic Solution
Blood In Hypertonic Solution. A hypertonic solution contains a higher concentration of solutes compared to another solution. Hypertonic dehydration occurs when an individual excretes too much.
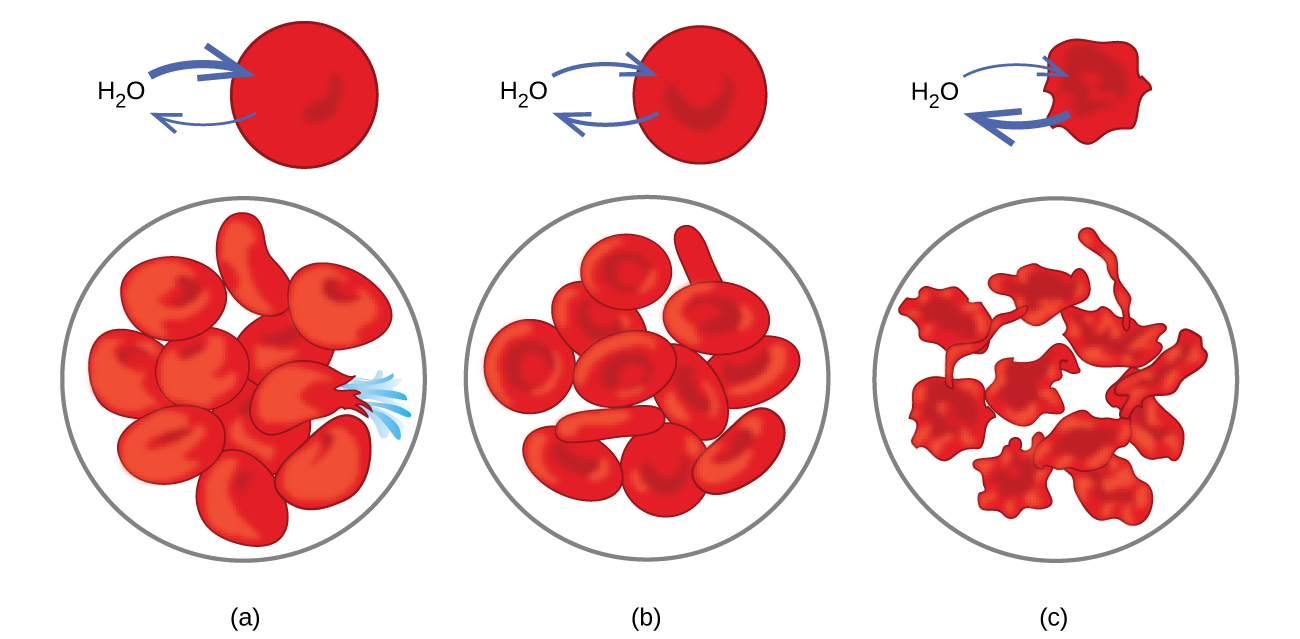
A hypertonic solution means that there is more salt in the solution or external environment than within the red blood cells. A hypertonic solution is a solution that has a greater concentration of solute compared to the cell and less water. Red blood cells placed in a hypertonic solution will shrink because osmosis will draw water out of the cells, causing their volume to decrease.
The potential contribution of hypertonic saline towards a coagulopathy during resuscitation should be studied in future clinical trials.
For example, hypertonic solutions are used for soaking wounds. If enough water is lost, the cell will take on a wrinkled or shriveled appearance. Hypertonic saline can also reduce the risk of organ failure.
Hypotonic solutions can cause the blood cell to burst from the pressure.
Hypertonic solutions cause blood cells to shrivel. Red blood cells placed in a hypertonic solution will shrink because osmosis will draw water out of the cells, causing their volume to decrease. Blood cells in isotonic solutions do not shrink or swell.
Generally, when water is excreted from the body, electrolyte (e.g., sodium) concentrations in the blood increase.
A solution that contains more dissolved particles (such as salt and other electrolytes) than is found in normal cells and blood. Scientists must describe cell contents compared to the environment. The outside solution has higher soluble concentration than inside the cell.
If the solution outside of the cell contains the same solute as the solution inside of the cell, the solution is isotonic.
If a cell is placed in an isotonic solution, there will be no net flow of water into or out of the cell, and the cell’s volume will remain stable. In red blood cells this is called crenation and the surface of the cells take on a scalloped appearance. A red blood cell will swell and undergo hemolysis (burst) when placed in a hypotonic solution.
If placed in a hypotonic solution, a red blood cell will bloat up and may explode, while in a hypertonic solution, it will.
If the solution inside of the cell has more solute than the solution outside of the cell, the solution is hypotonic. A hypertonic solution means that there is more salt in the solution or external environment than within the red blood cells. Most cells appear shrunken or crenate and less biconcave than normal red blood cells.
Demikianlah Artikel Blood In Hypertonic Solution
Anda sekarang membaca artikel Blood In Hypertonic Solution dengan alamat link https://infobreaking-03.blogspot.com/1969/12/blood-in-hypertonic-solution.html


0 Response to "Blood In Hypertonic Solution"
Post a Comment