Judul : Examples Of Hypertonic And Hypotonic Solutions
link : Examples Of Hypertonic And Hypotonic Solutions
Examples Of Hypertonic And Hypotonic Solutions
Examples Of Hypertonic And Hypotonic Solutions. These two solutions will together form a single solution due to the passive exchange of ion and other molecules and gets equalized or isotonic in nature. An example of a hypertonic solution is the interior of a red blood cell compared with the solute concentration of fresh water.
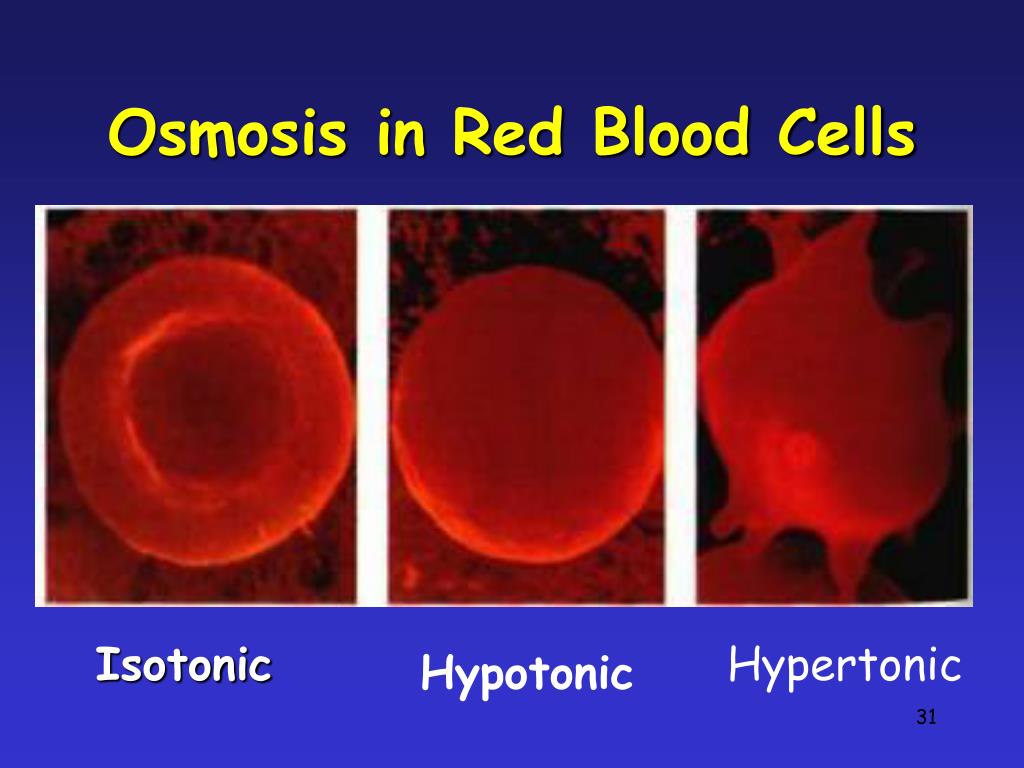
The clinical physiology chapter is now broken into several short chapters. Hypotonic, hypertonic, and isotonic solutions are the three kinds of solutions characterised by tonicity. Crystalloids are the solutes that form isotonic, hypotonic and hypertonic solutions.
Example of a hypotonic solution and hypertonic solution.
In the case of a reversal, the extracellular fluid with higher osmolarity than the cell’s cytoplasm will be hypertonic. When a hypertonic solution placed in an environment of low salt concentration or in a hypotonic solution then both solutions flow up and mix up with each other. The best example of a hypertonic solution would be the oceans because the solutes (salts) outside of the cells are greater than inside of the cells.
The solution may be pure water or the solution may be water with a solute dissolved in it, or any such solution.
Some examples of isotonic solutions are sodium chloride (ns) or lactated ringers (lr) solution. A cell becomes shrivelled in a hypertonic solution, while a cell gets swelled in a hypotonic solution. Crystalloids are the solutes that form isotonic, hypotonic and hypertonic solutions.
Hypotonic solutions have fewer solutes (in terms of concentration) than other solutions.
The osmotic pressure of a hypotonic solution is lower than that of the solution being compared to. When you put a mushroom into a solution and its cells bloat, you know the solution is hypotonic. Fresh water is a hypotonic solution for saltwater fish.
For example, if you swim in an ocean, you can observe that your body tends to dry out due to the solvent (ocean) having a greater amount of solutes than the inside of your body.
For example, say if we place a cell in a solution, which is the example we will use for all the various solutions. An example of a hypertonic solution is the interior of a red blood cell compared with the solute concentration of fresh water. A hypotonic solution example is salt water.
Common examples of hypertonic solutions are d5 in 0.9% normal saline and d5 in lactated ringers.
Some examples of hypotonic solutions include anything that has more water and less solute compared to the cells: Examples of these are nacl and / or sugar in different concentrations (osmolarities) or in different proportions. The salt is the solute, and the water.
Demikianlah Artikel Examples Of Hypertonic And Hypotonic Solutions
Anda sekarang membaca artikel Examples Of Hypertonic And Hypotonic Solutions dengan alamat link https://infobreaking-03.blogspot.com/1969/12/examples-of-hypertonic-and-hypotonic.html


0 Response to "Examples Of Hypertonic And Hypotonic Solutions"
Post a Comment