Judul : Hypertonic Solution Concentration
link : Hypertonic Solution Concentration
Hypertonic Solution Concentration
Hypertonic Solution Concentration. The outside solution has higher soluble concentration than inside the cell. A hypertonic solution is a solution that contains more solute than the cell which is placed in it.
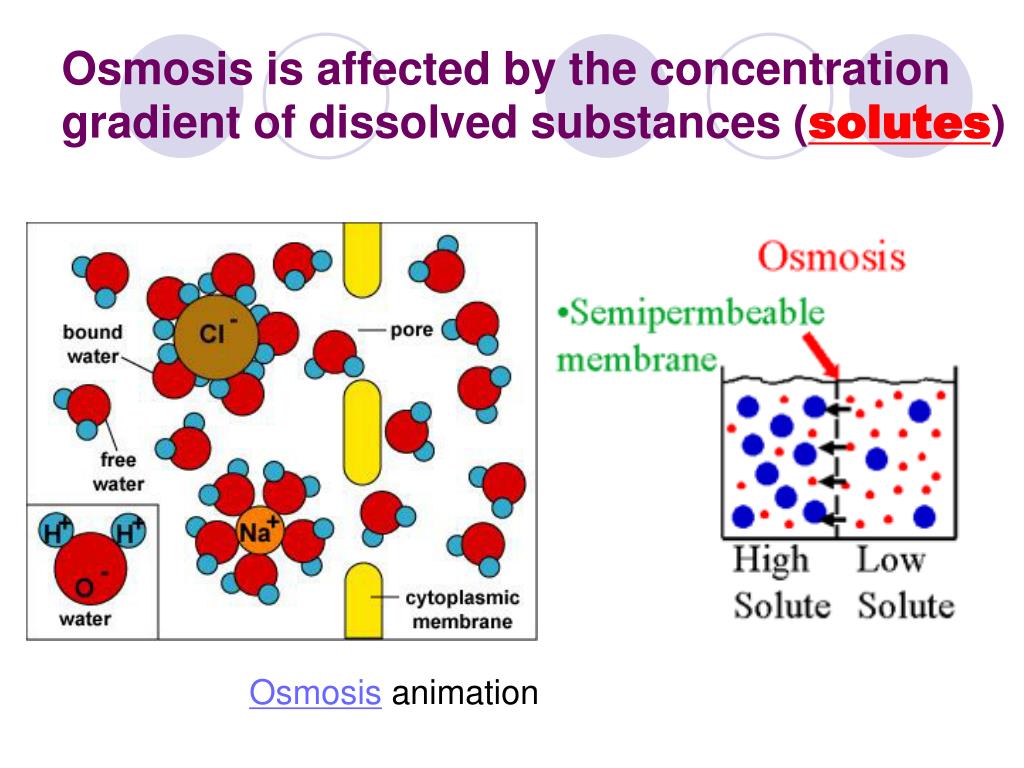
When a hypertonic solution is administered intravenously, fluid shifts from the interstitial and intracellular spaces into the bloodstream to dilute the electrolytes. If a cell is placed in a hypertonic solution, the cell will shrink due to water osmotically moving out. A hypertonic solution is one which has a higher solute concentration than another solution.
If a cell with a nacl concentration of 0.9% is placed in a solution of water with a 10% concentration of nacl, the solution is said to be hypertonic.
The opposite solution with a lower concentration is known as the hypotonic solution. A hypertonic solution contains a higher concentration of solutes compared to another solution. The solute concentration of the solution outside the cell is lower than that of the fluids inside the cell.
Scientists must describe cell contents compared to the environment.
Hypertonic saline is an osmotic agent uses to reduce the effects of secondary brain injury in patients with traumatic brain injury (tbi). A hypertonic solution is a solution that contains more solute than the cell which is placed in it. Yes, the hypertonic solution contains a higher concentration of sodium ions than the blood.
The outside solution has higher soluble concentration than inside the cell.
A solution outside of a cell is called hypertonic if it has a greater concentration of solutes than the cytosol inside the cell. So when you give a hypertonic solution to someone who has hyponatremia, you are essentially mixing a liquid with high concentration of na (the hypertonic solution) with a liquid having a low concentration of na (the hyponatremic blood). A hypertonic solution is defined as one having a high osmotic pressure when compared to other solutions.
In the context of biology, when two aqueous solutions are separated by a cell membrane, if the concentration of solute is greater outside the cell than inside the membrane, the solution is called hypertonic.
This leads to water leaving the cell and flowing into the solution around it. The solute concentration in the solution outside the cells is higher than in the fluids inside the cells. An example of a hypertonic solution is the interior of a red blood cell compared with the solute concentration of fresh water.
It is defined as the solution with a high solute concentration outside than the fluids inside the cell, and a high water concentration inside the cell than the surrounding fluid.
When a hypertonic solution is administered intravenously, fluid shifts from the interstitial and intracellular spaces into the bloodstream to dilute the electrolytes. A hypertonic solution is one that has a higher solute concentration outside the cell than inside. In biology, the tonicity of a solution usually refers to its solute concentration relative to that of another solution on the opposite side of a cell membrane;
Demikianlah Artikel Hypertonic Solution Concentration
Anda sekarang membaca artikel Hypertonic Solution Concentration dengan alamat link https://infobreaking-03.blogspot.com/1969/12/hypertonic-solution-concentration.html


0 Response to "Hypertonic Solution Concentration"
Post a Comment