Judul : Hypertonic Solution Nursing
link : Hypertonic Solution Nursing
Hypertonic Solution Nursing
Hypertonic Solution Nursing. If you haven’t already seen the explanation of results from my osmosis experiment, make sure to check that out first.it’s not only interesting,. I put this chart here to help you visualize why the iv solution is hypertonic, hypotonic or isotonic.
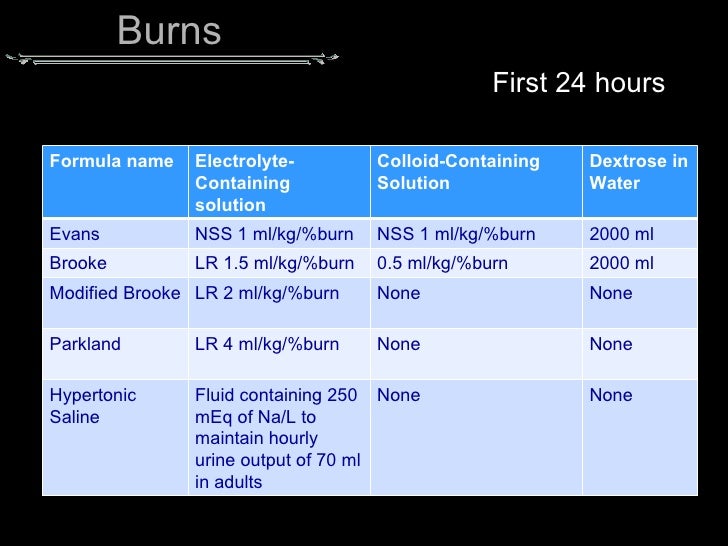
Vital signs, edema, auscultate for heart and lung sounds. In nursing school and on the nclex exam, you will be required to know what type of iv fluids are considered isotonic, hypotonic, and hypertonic. Low sodium, low glucose, etc.) hypertonic solutions can also draw water from the icf to the ecf which can be useful for clients with edema.
Additionally, if hypertonic solutions with sodium are given, the patient’s serum sodium level should be closely monitored.
It’s isotonic with our blood. I put this chart here to help you visualize why the iv solution is hypertonic, hypotonic or isotonic. Low sodium, low glucose, etc.) hypertonic solutions can also draw water from the icf to the ecf which can be useful for clients with edema.
Hypervolemia or fluid overload, swelling in the face, arms, and legs, hypertension, difficulty of breathing, signs of hyperglycemia, and any discomfort in the body.
So it’s going to be things such as 1/2 normal saline (0.45% sodium chloride), ¼ normal saline (0.225% ns), d5 in half normal saline (5% dextrose and 0.45% sodium chloride) something that might be used in dka, for instance, as well as d5. Isotonic, hypotonic, and hypertonic solutions the principles for the use of isotonic, hypotonic, and hypertonic solutions are rooted in the goal of equilibrium through osmosis.when administering a fluid intravenously to a patient, the ratio of fluid to electrolytes in the solution and in the patient’s bloodstream will impact the body’s reaction. Whereas d5 + ringer’s is a hypertonic solution and it has 361 particles.
3 ways surgical procedures may affect fluid balance within the body the definition of hypotonic.
Most likely given to counter the effects of fluid overload or pulmonary edema Hypotonic, hypertonic, & isotonic iv solution quiz for nursing students & nclex exam. Hypotonic solutions or anything, essentially less than 0.9% normal saline.
Hypertonic solutions cause the cell to become skinny because of the fluid that escapes from it.
These solutions are generally utilized when clients have low lab values (e.g. If you haven’t already seen the explanation of results from my osmosis experiment, make sure to check that out first.it’s not only interesting,. In this article, i give you an easy overview of each solution and how they work on the cellular level.
This fluid & electrolyte quiz is designed to test your knowledge on hypotonic, hypertonic, and isotonic solutions.
Osmosis results part 1 (from left to right) hypotonic solution, isotonic solution, hypertonic solution. D5w 1/2 ns (d5w 0.45% ns) d5w 0.09% ns (d5w ns) 3% sodium chloride (3% nacl) 3% nacl hypertonic solution’s given for severe hyponatremia or cerebral edema. It was super hyper, it danced around everywhere, and now it’s lost all it’s fluid and weight and its a skinny little cell.
Demikianlah Artikel Hypertonic Solution Nursing
Anda sekarang membaca artikel Hypertonic Solution Nursing dengan alamat link https://infobreaking-03.blogspot.com/1969/12/hypertonic-solution-nursing.html


0 Response to "Hypertonic Solution Nursing"
Post a Comment