Judul : If Cells Are Placed In A Hypertonic Solution
link : If Cells Are Placed In A Hypertonic Solution
If Cells Are Placed In A Hypertonic Solution
If Cells Are Placed In A Hypertonic Solution. Hyper means more, meaning that the solution that the cell is placed in contains more solute than the solution inside of the cell. The osmolarity inside the cell is greater than the osmolarity outside the cell, so the cell shrinks the cell swells because the osmolarity.
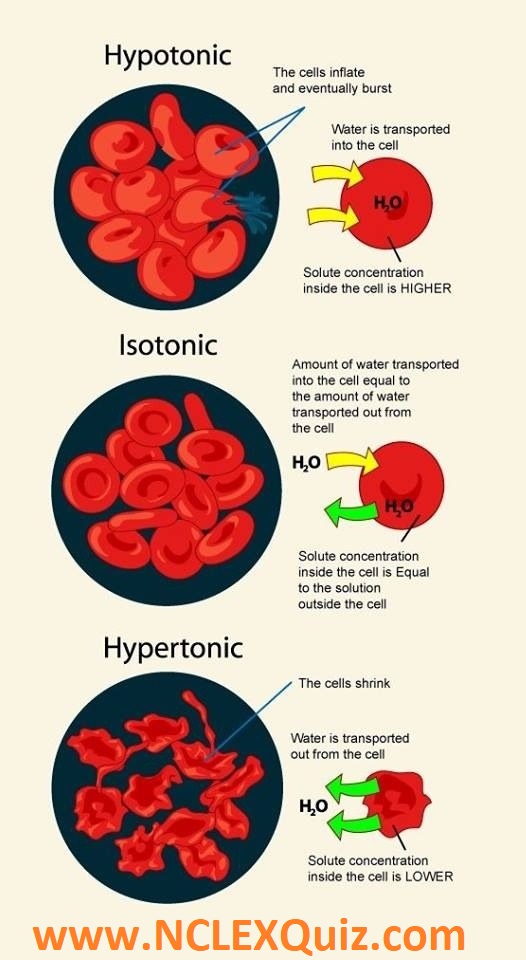
The pressure inside the cell rises until this internal pressure is equal to the pressure outside. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until both solutions are isotonic. Therefore, a hypertonic solution has more solutes than the intracellular environment, so water will leave the cell to try to achieve equilibrium.
For example, if you place a cell in a salt solution, the salt solution is more hypertonic (more concentrated) than the cell plasma.
The cells will shrink at first, but will later reach equilibrium This is because, during osmosis, water moves from a region of its higher concentration to a region of its low concentration. The opposite solution with a lower concentration is known as the hypotonic solution.scientists must describe cell contents compared to the environment.
In red blood cells this is called crenation and the surface of the cells take on a scalloped appearance.
This causes a lack of structure for the plant and causes it to wilt, or become flaccid. The osmolarity inside the cell is greater than the osmolarity outside the cell, so the cell shrinks the cell swells because the osmolarity. Cells placed in a hypertonic solution will shrink due to water loss by the cell.
A hypertonic solution is a solution that contains more solute than the cell which is placed in it.
Question 3 if cells are placed in a hypertonic solution containing a solute to which the membrane is impermeable, what could happen? A high amount of water loss can be. The terms hypertonic and hypotonic often confuse students because they neglect to account for the frame of reference.
A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane.
Water always moves from a region of low to high osmolarity. The pressure inside the cell rises until this internal pressure is equal to the pressure outside. Water tends to move from a solution of low tonicity to a solution with high tonicity.
If a cell is placed in a hypotonic solution, water will move into the cell.
With this, there is a net movement of water from inside to outside initiating water to lose from the cytoplasm and vacuole causing the cell to shrink due to osmosis and achieve equilibrium. Anatomy and physiology questions and answers. An hypertonic solution has a high content of solutes dissolved, which makes it a solution with high tonicity.
Demikianlah Artikel If Cells Are Placed In A Hypertonic Solution
Anda sekarang membaca artikel If Cells Are Placed In A Hypertonic Solution dengan alamat link https://infobreaking-03.blogspot.com/1969/12/if-cells-are-placed-in-hypertonic.html


0 Response to "If Cells Are Placed In A Hypertonic Solution"
Post a Comment