Judul : Rbc In Hypertonic Solution
link : Rbc In Hypertonic Solution
Rbc In Hypertonic Solution
Rbc In Hypertonic Solution. A solution that has a lower solute concentration than is present in cells is said to be a hypotonic solution. Red blood cells (rbcs) have been placed in three different solutions:
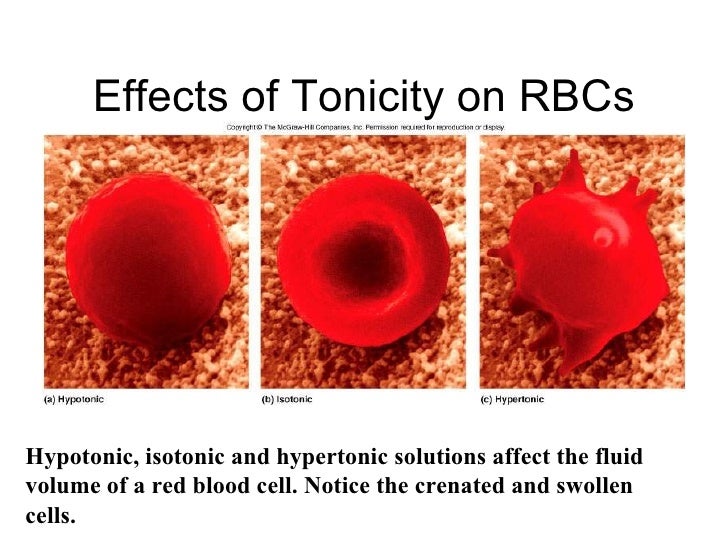
When rbc is placed in hypertonic solution, crenate form of rbc appears due exosmosis. If the red blood cells take in too much water, cytolysis can occur. What happens when rbc are placed in hypertonic solution endosmosis or exosmosis?
Hypotonic solutions have lower osmotic pressure than red blood cells, causing the cells to take in additional water.
This causes the cells to shrink. When rbcs are positioned in a hypertonic salt answer, water strikes from contained in the cell to the surface by osmosis. Such solution which solute concentration is equal to intracellular solute concentration is called isotonic solution.
Click on hashtags above the video title or the links given below to watch my other related videos.total wbc count:
All of this additional water moving by osmosis into the blood vessels can quickly cause high blood pressure (and all the complications that come with it) if not done carefully. Red blood cells are suspended in a 0.85% saline solution. What will happen to the red blood cell if it is placed in a solution that is 99.3% water and 0.7% salt is the cell hypertonic or hypotonic to the solution?
What is the condition of the rbc in solution b?
If placed in a hypotonic solution, a red blood cell will bloat up and may explode, while in a hypertonic. Red blood cells (rbcs) have been placed in three different solutions: If the red blood cells take in too much water, cytolysis can occur.
The red blood cell will lose water and will shrink.
When two solutions are in contact, solute or solvent moves until the solutions reach equilibrium and become isotonic with respect to each other. When rbc is placed in hypertonic solution, crenate form of rbc appears due exosmosis. The phenomenon is called crenation.
If a cell with a nacl concentration is placed in a solution of distilled water, which is pure water with no dissolved substances it, the solution.
A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. A hypotonic solution is a solution that contains less solute than the cell which is placed in it. The average sodium value for the red blood cells of twenty normal pregnant women was 16.6 mg.
Demikianlah Artikel Rbc In Hypertonic Solution
Anda sekarang membaca artikel Rbc In Hypertonic Solution dengan alamat link https://infobreaking-03.blogspot.com/1969/12/rbc-in-hypertonic-solution.html


0 Response to "Rbc In Hypertonic Solution"
Post a Comment